-
Bond Length and Bond Order in One of the Shortest Cr−Cr Bonds
G. La Macchia, F. Aquilante, V. Veryazov, B.O. Roos and L. Gagliardi
Inorganic Chemistry, 47 (24) (2008), p11455-11457


DOI:10.1021/ic801537w | unige:3563 | Abstract | Article HTML | Article PDF
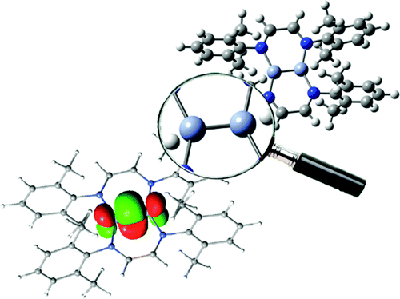
Multiconfigurational quantum chemical calculations on the R-diimines dichromium compound confirm that the Cr−Cr bond, 1.80 Å, is among the shortest CrI−CrI bonds. However, the bond between the two Cr atoms is only a quadruple bond rather than a quintuple bond. The reason why the bond is so short has to be attributed to the strain in the NCCN ligand moieties.